A Biomimetic Immune System ™
Restore Immunity. Repair Tissues. Extend Healthspan.
Infections Drive Almost Every Major Cause of Age Related Decline
Maxwell was founded using artificial intelligence to analyze the proteomics of how the body heals itself, and what peptides in the body decline with age leading to the hallmarks of biological aging.
The conception of our drug development technology was incubated by the US Defense Advanced Research Projects Agency (DARPA), the US Department of Energy (DOE), the National Institutes of Health (NIH), and invented by a team of academic scientists.
There is scientific consensus that biological aging is driven by the weakening of the immune system - called immunosenescence. Maxwell’s Biomimetic Immune System ™ technology may reverse aspects of immunosenescence, and may be a key biodefense asset regional governments use to stop pandemics.
Armoring Civilization — a Pathogen-Agnostic Biodefense Shield
Almost universally, the greatest past civilizations have collapsed due to pandemics.
To name just a few: the Egyptians, Minoans, Aztecs and Mayans.
Our civilization has become fragile due to the last pandemic. We need a universal biodefense that works against all known pathogens of pandemic concern: extremely broad spectrum, easy to use, nontoxic therapeutics that do not need refrigeration and do not allow pathogens to develop resistance.
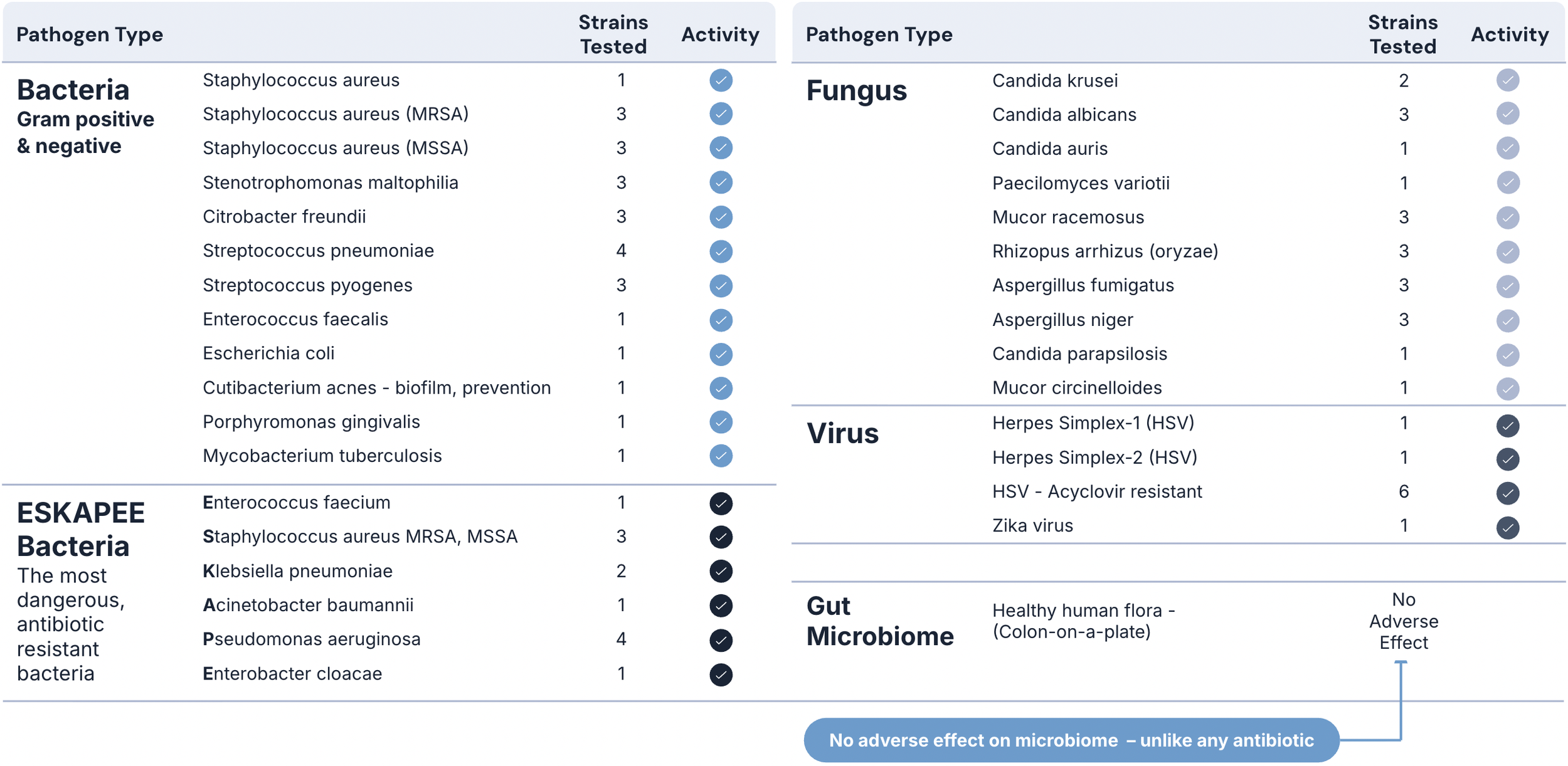
Maxwell’s Claromer® drug candidates have been shown in preclinical studies to be highly potent against all tested AMR bacteria, fungi, mold, yeast, as well as pan-coronavirus, pan-influenza, pan-herpes, RSV, Rhinovirus and can completely suppress the Ebola virus.
Our first lead drug candidate, MXB-22,510, has the potential to replace many antifungals and antibiotics, as well as provide a front line therapy for many types of viral infections.
MXB-22,510: Our Strategic Beachhead Molecule - “One Drug for Many Bugs"
A Biomimetic Immune System ™
Maxwell’s First-in-Class CLAROMER® brand drug-discovery platform produces molecules that mimic the pathogen-agnostic core peptide of the innate immune system - Human Cathelicidin Antimicrobial Peptide - that is able to selectively target all kinds of pathogens without the use of antibodies, white blood cells, without feeling sick. That’s how your innate immune system works.
We are pioneering the future of anti-infective therapeutics with our innovative Claromer® platform, inspired by the remarkable potential of innate immune peptides and advanced through cutting-edge peptidomimetic technology. Recently, peptides have emerged as powerful drugs, blending the precision of antibodies with the versatility of small molecules, offering unmatched specificity and selectivity for their targets. With over 80 peptide-based therapies already FDA-approved (as of 2025) and many more in development, their role in medicine is undeniable—acting as hormones, growth factors, neurotransmitters, and anti-infective agents while binding to cell receptors with high affinity.
Yet, challenges like poor cellular permeability and metabolic instability limit their potential. This is where Maxwell Biosciences steps in with a game-changing solution: peptide-like small molecules called “peptoids” in academic literature, specifically N-substituted glycine oligomers, which form the backbone of our Claromer® family of Bio small molecule therapeutics. Unlike natural peptides, our Claromers resist proteolytic degradation, boast exceptional thermal stability, and offer superior bioavailability and cell permeability. They mimic the antimicrobial prowess of natural peptides, such as the Human Cathelicidin LL-37, while overcoming pharmacological hurdles with lower manufacturing costs and a non-immunogenic profile.
Peptoids, as a class, are revolutionizing drug development by enabling access to previously "undruggable" spaces, such as protein-protein interactions. Synthesized efficiently on solid support, they allow for diverse functional modifications and fold into precise secondary structures for a range of biological applications. Their stability against proteases, tolerance to extreme conditions, and lipophilic nature make them ideal for therapeutic and diagnostic innovation. At Maxwell, we’ve harnessed these advantages to develop Claromers with potent, broad-spectrum activity against multi-drug resistant pathogens, including fungi and viruses, through novel mechanisms that deter resistance.
Our research builds on recent advances in peptoid applications—from antimicrobial agents to anticancer inhibitors—driving forward a new era of biostable, cost-effective therapeutics. Whether designed de novo or as peptide-peptoid hybrids, Claromers are engineered to maximize efficacy while minimizing the loss of bioactivity often seen in structural modifications. With Maxwell Biosciences, we’re not just mimicking nature; we’re enhancing it to combat the global threat of infectious diseases with unparalleled precision and durability.
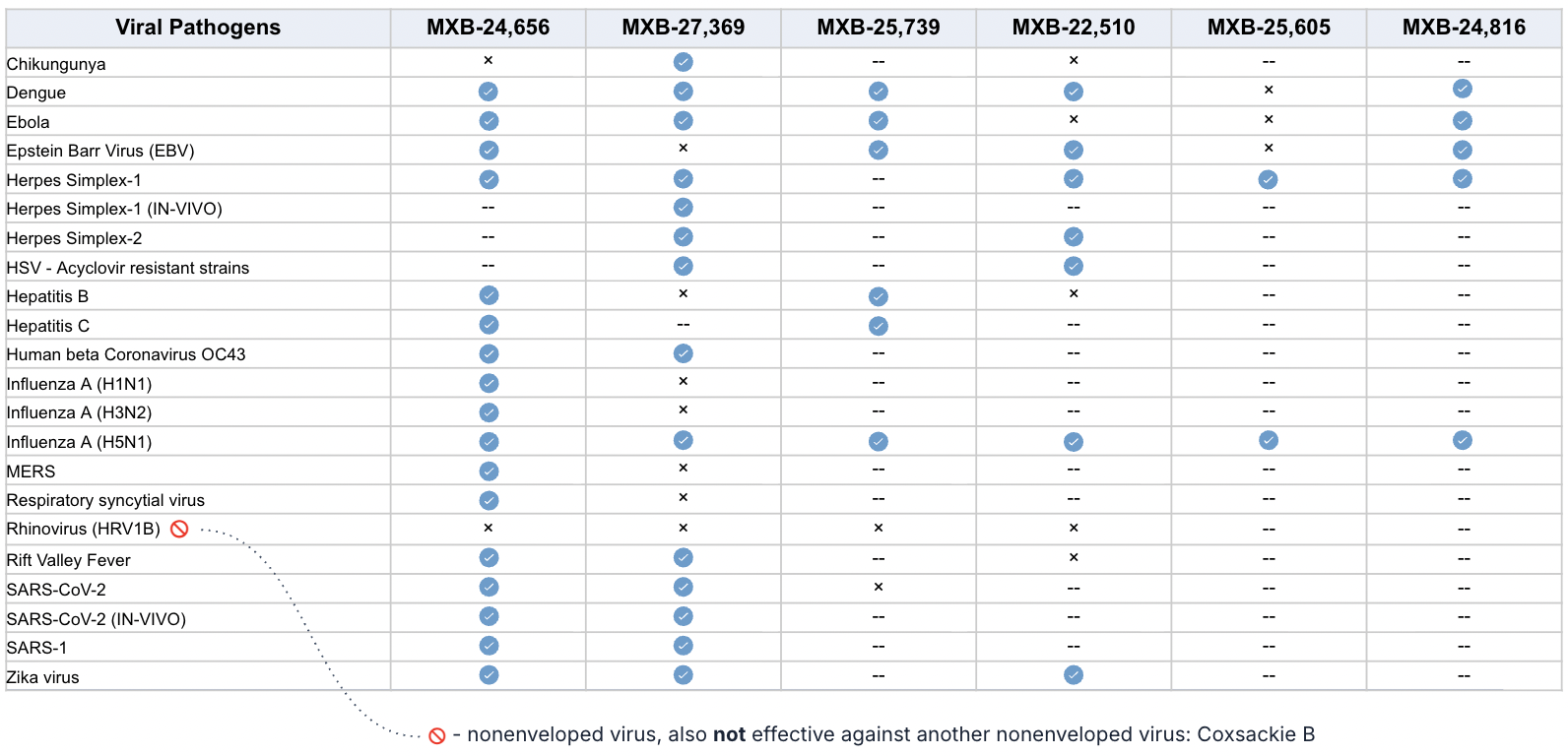
How Many Viruses?
Preclinical studies show the Claromer technology is effective against pretty much every enveloped virus tested so far (even some non-enveloped viruses) at extremely low doses.
NIAID performed animal studies that show the technology is highly effective at destroying viruses, and also modulating the immune system to keep it from going into a “cytokine storm” inflammatory overdrive.
Human Trials
If this works in humans, it changes everything.
Human trials are planned for 2026 - with encouraging FDA feedback already received. Our vision is to bring this to the world, all while making therapies much more affordable for patients.
The Team
The team includes the former leadership of Shire Pharmaceuticals US R&D program: highly successful industry veterans who are responsible for over $30 billion in revenue at Shire, and have collectively commercialized hundreds of drugs; including multi-billion dollar blockbusters like Adderall XR, Carbatrol, Equetro and Claritin. We’re heading into FDA human trial interactions with perhaps the world’s best team. More About the Team
Acknowledgements
In gratitude, we extend our humble thanks to the multitude of independent researchers who have donated their labs and efforts to challenging and confirming our Claromer “peptoid” science with over a decade of research - much of which has been published in over 250 peer-reviewed articles in respected scientific journals.
About Us
Founded in 2016, Maxwell is a preclinical stage biotechnology company targeting viruses, bacteria, fungi, and biofilm formations, including those that cause many currently untreatable infectious diseases. Stanford and NYU scientists invented a new class of broad spectrum anti-infective therapeutics that attack the structure of most infectious disease bugs.
Maxwell’s CLAROMER® branded platform develops a diverse family of biostable, low molecular weight compounds which are functional mimics of peptides (“peptidomimetics”) at roughly one-quarter the size, and are distinct from macromolecules due to their ability to easily penetrate target membranes.
Maxwell’s vision is to create health for the world safely and affordably.
Technology
Maxwell’s CLAROMER® brand anti-infectives platform includes a diverse library of small molecule structures demonstrated to be well tolerated in animals and human tissues - and nontoxic and anti-inflammatory at over 100 times relevant therapeutic dosages. Our lead drug candidates destroy a broad spectrum of pathogens, as shown in preclinical animal studies. Triple confirmation by many independent labs using super resolution fluorescent microscopy, soft X-Ray tomography, and electron microscopy imaging of bacterial, viral and fungal structures and functions reveals the mechanism of action: highly selective targeting of pathogen-specific phospholipids that pathogens need in order to be able to infect human cells. Claromer molecules have also been shown in preclinical studies to be well tolerated in pseudostratified human lung epithelial mucosal tissues. Maxwell’s technology is protected by an umbrella of granted patents.
Development Programs
Nasal Spray. A nasal spray therapeutic for unmet medical needs driven by airborne pathogens is going into human trials next year, has encouraging feedback from the FDA, and has successfully passed animal studies sponsored and performed by NIAID.
Oral. An oral dosage form is in early stages for difficult to treat gastrointestinal indications triggered by infectious pathogens and microbiome imbalance.
Maxwell’s data has been generated by the following institutions:
Stanford University, National Institute of Allergy and Infectious Diseases (NIAID), Infectious Disease Institute Tokyo, New York University, NIH Rocky Mountain Lab, University of Texas Medical Branch, ProDigest, Texas A&M, Charles River Labs, University of Louisville, University of Otago New Zealand, Baylor College of Medicine, and Northwestern University